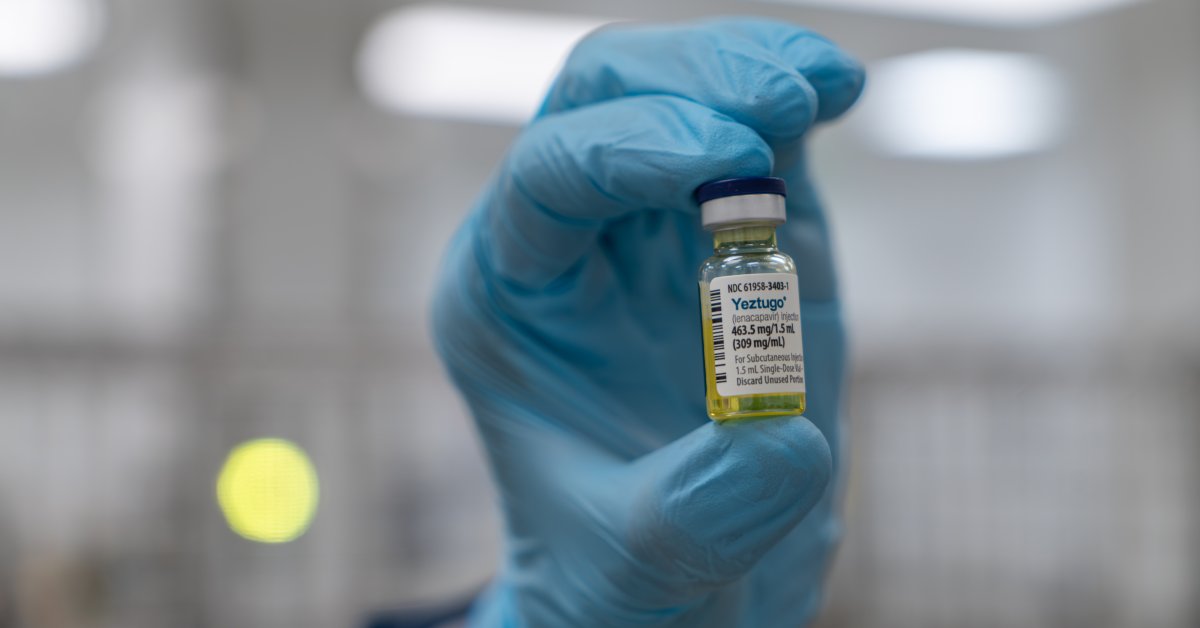
On June 18, the U.S. Meals and Drug Administration (FDA) accepted the primary drugs to forestall HIV that solely must be taken twice a 12 months. People who find themselves at excessive danger for HIV can now take the injection—known as lenacapavir and offered as Yeztugo—simply as soon as each six months.
The approval is a milestone within the struggle towards HIV and will rework the epidemic. Whereas anti-HIV drug therapies have helped hundreds of thousands of individuals suppress the virus to undetectable ranges in order that they don’t unfold it to others—and have additionally allowed people who find themselves HIV-negative to keep up their standing when used to forestall an infection—a routine of each day tablets signifies that compliance, and subsequently effectiveness, is commonly not as sturdy correctly.
In two research, scientists at Gilead, which developed lenacapavir, confirmed that the drug was 100% efficient at defending cisgender ladies from changing into HIV optimistic as in comparison with each day oral tablets (known as PrEP, quick for pre-exposure prophylaxis). In males who’ve intercourse with males and gender-diverse folks, the drug was 96% efficient.
Learn Extra: ‘That is About Youngsters’s Lives’: Gavi’s CEO Makes the Case for Funding the International Vaccine Alliance
“Lenacapavir utilized by itself for prevention is a big breakthrough,” says Dr. David Ho, professor of microbiology, immunology, and drugs at Columbia College who pioneered combining anti-HIV medication to suppress the virus and its means to mutate to turn into proof against therapy. “Its potential is nice in curbing the epidemic.”
However advocacy teams and international AIDS organizations elevate issues about whether or not that potential shall be absolutely realized, given current cuts to U.S.-supported packages for HIV therapy and prevention around the globe.
From therapy to prevention
Lenacapavir was accepted by the FDA in 2022 to deal with folks with HIV whose virus has turn into proof against different antiviral medication. As they had been creating that therapy, Gilead’s scientists observed that lenacapavir had two essential properties that would make it doubtlessly helpful in stopping HIV as properly: its means to stay within the physique for an extended time than different antiviral medication, and its energy to intervene with a number of steps within the course of that the virus makes use of to make copies of itself.
“We noticed a incredible impact after a single injection,” says Tomas Cihlar, senior vice chairman of virology at Gilead. “Mainly, it protected non-human primates from buying HIV. That’s once we realized we actually wanted to get full pace and full power behind the prevention thought.”
Learn Extra: TIME100 Well being: Tomas Cihlar and Wesley Sundquist
However as a result of folks taking a drug to forestall an infection are HIV damaging, “the bar for security for individuals who don’t have the illness is kind of excessive,” says Jared Baeten, vice chairman of HIV growth at Gilead. “Nonetheless, primarily based on all of the pharmacology, science, and demonstrated antiviral exercise and security within the HIV therapy sphere, by the top of 2020 we made the choice to maneuver lenacapavir into prevention,” he says.
Whereas it’s the identical drug, when docs use lenacapavir to deal with HIV, they usually mix it with different medication to restrict HIV’s potential for creating resistance. However to forestall illness in people who find themselves HIV damaging, lenacapavir can be utilized alone, since there isn’t already an actively reproducing inhabitants of virus within the physique.
How lenacapavir may quash HIV vaccine efforts
Lenacapavir will not be an HIV vaccine, however its impact in stopping an infection is just like one. Vaccines enlist and prepare the immune system to acknowledge and goal pathogens like viruses, so the physique turns into a manufacturing unit for producing the suitable defenses to struggle infections. Lenacapavir’s means to fend off an infection comes from having circulating ranges of the drug within the physique to struggle off any virus which may enter.
Whether or not it is the immune system or the drug, the impact may be very related—which is a big advance. Within the greater than 40 years since HIV was found, scientists haven’t been capable of develop a vaccine towards it. “To date the candidate vaccines don’t present this type of promise for stopping HIV an infection,” says Ho. “We’re nowhere near an efficacious vaccine.”

With lenacapavir accepted to forestall HIV, the bar for creating a vaccine turns into even increased. It may very well be ethically tough to justify asking folks to take a placebo to ascertain the results of a vaccine, since each oral PrEP and now lenacapavir as PrEP are so efficient in defending towards HIV an infection. Depriving these in a vaccine trial from taking lenacapavir by assigning them to obtain a placebo could be problematic. “This may take a little bit of the wind out of the sails of vaccine analysis, as a result of there’s something so efficient at stopping HIV an infection,” says Ho.
The way forward for HIV therapy and prevention
The long-acting nature of lenacapavir represents a brand new route for anti-HIV medication that would make stopping infections extra tenable and accessible to extra folks, says Hui Yang, head of provide operations of the International Fund to Combat HIV, TB, and Malaria. “We discovered from decades-long expertise that in prevention packages, adherence is an enormous challenge. And that’s a facet we hope to handle with the introduction of lenacapavir for PrEP.” The International Fund’s objective is to get two million extra folks in prevention packages over the following three years, and lenacapavir may speed up reaching that focus on.
Learn Extra: Tedros Adhanom Ghebreyesus: International-Well being Architect
However the shot nonetheless should be administered by a well being care supplier, and other people want to check damaging for HIV earlier than receiving every shot. These are hurdles for lots of the most weak populations in low- and middle-income international locations, a lot of whom are younger adults. To additional improve entry, Yang says, a type of lenacapavir that folks may inject themselves with twice a 12 months could be much more applicable for such settings. “It may turn into one thing like an insulin injection that folks can do themselves,” she says.
Gilead is engaged on a once-a-year model of lenacapavir that will lower down on the necessity for a number of clinic visits.
The unrealized potential of lenacapavir
Whereas the advance is scientifically thrilling, the drug might take years and even a long time to considerably curb the worldwide HIV epidemic. “We simply constructed the most effective airplane on the planet, however sadly tore up all of the runways,” says Kevin Frost, CEO of amfAR, the Basis for AIDS Analysis. “Lenacapavir is coming on the worst second within the final 30 years of the AIDS epidemic. We’re seeing ourselves time touring again a long time due to the dismantling of the infrastructure across the therapy and prevention of HIV.” Cuts to USAID, PEPFAR, and the Nationwide Institutes of Well being “imply lenacapavir won’t ever have a shot popping out of the gate. The very structure that would ship lenacapavir on a worldwide scale to be transformative is being dismantled.”
Whereas Gilead wouldn’t specify a worth for lenacapavir, an organization spokesperson stated it might probably be “in keeping with present branded PrEP choices.” Nonetheless, that may very well be out of attain for these in decrease revenue international locations who may gain advantage essentially the most, says Frost.
Addressing price and different points associated to entry shall be vital to realizing the complete potential of the drug. Within the U.S., states that provide and canopy PrEP choices have reported a 38% lower in new infections, whereas states that don’t make PrEP as obtainable noticed a 27% improve in infections from 2012 to 2022, in response to a current report printed within the Lancet HIV. Gilead has negotiated royalty-free licensing offers with six generic producers to fabricate lenacapavir for prevention for 120 low- and middle-income international locations.
“Hopefully in these locations the place they’ve that sort of manufacturing capability, we’ll see low cost, cheap lenacapavir,” says Frost—however on condition that the traditional distribution channels are newly gutted packages like USAID and PEPFAR, “I nonetheless anticipate entry shall be terribly restricted.”
Correction, June 20
The unique model of this story misstated how a lot safety the teams in two research skilled after taking the drug lenacapavir. Cisgender ladies skilled 100% safety from an infection, not 96%, and males who’ve intercourse with males and gender-diverse folks skilled 96% safety, not 100%.